16 years of serving high-end industries
with high-quality products |
|
|
Crystal growing is a technological process of crystallization carried out to obtain single crystals or films of different materials. In industry and research laboratories crystals can be grown from melts, vapors, solutions, solids, gels, or synthesized chemically, under high pressure, by way of electrolytic crystallization etc.
Growth from the Melt
At present methods of this kind are the most popular crystallization processes used in industry. Most of semicondustors, metals, oxides, tungstates, vanadates, niobates, chalcogenides and halogenides are grown from the melt. Some of these crystals consist of more than five components. Each method of growth from the melt has its pros and cons and since there is quite a big choice of them you have an opportunity to compare them and select the one most suitable for obtaining a single crystal with particular properties.
|
Sapphire, ruby, spinel
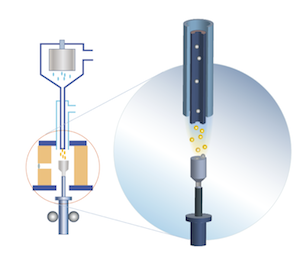
August Verneuil was one of the first to introduce crystal growing technologies. Verneuil process, also known as flame fusion, has been used for growing corundum crystals (sapphire, ruby and spinel) since 1904. In a Verneuil furnace alumina powder gets fused in a hydrogen-oxygen flame and then falls down onto the molten surface of an oriented seed crystal. The method makes it possible to grow crystals of up to 60 mm in diameter which are usually used in watch industry and jewelry. |
| Sapphire, silicon, CdWO4, NaBi(WO4)2, ruby, YAG, GaAs, InP
The method was first introduced in 1918. The raw material is charged into a refractory crucible and is heated until it all melts down. Then a seed crystal shaped as a thin rod of a few mm in diameter is mounted onto a seed crystal holder and is dipped into the melt. All through the process the seed crystal holder is being cooled. The column of the melt which connects the grown crystal with the melt is maintained by surface tension force and this column forms a meniscus between the surface of the melt and the growing crystal. The solid-melt interface, or crystallization front, gets over the surfaces of the melt. The temperature of the melt and the conditions of the abstraction of heat from the seed crystal determine how high the crystallization front gets. When the end of the seed partially melts the seed is pulled out of the melt together with the crystallized material. At the same time the crystal is being rotated. It helps to keep the melt blended and to maintain the same temperature at the crystallization front. As a result of heat abstraction an oriented single crystal starts growing on the seed. The diameter of the crystal can be controlled by adjusting the speed of growth and the temperature of the melt.
The main advantages of pulling from the melt is that the crystal is grown in an open space, so the crystal does not come in contact with the crucible material, and it is easy to control the growing process and change the diameter of the crystal. A disadvantage consists in the difficulty to maintain chemical homogeneity of the crystal along the growth direction.
Czokhralski method is used in industry to grow semiconductor materials (silicon, GaAs, InP etc.), dielectric materials and jewelry crystals (sapphire, ruby, YAG etc.)
The pulling technology can vary depending on the type of material crystallized and the desired result. Crystals can be pulled in vacuum and in inert gas under different pressure, with or without a container.
To grow single crystals containing fugitive components a modified technology is used: the melt is covered by a soft fluxing material with a lower density. The crystallization is carried out in inert gas under high pressure (10MPa).
A low-gradient Czokhralski method was developed in the 1980's to obtain single crystals of compound tungsten and molybdenum oxides. It is adapted to grow scintillating crystals, such as CdWO4 and NaBi(WO4)2. The technique requires a long crucible for the melt and a resistance oven with three independent temperature control loops. This configuration gives no possibility to maintain visual control and it is important to carry out automatic weight control during the process. |
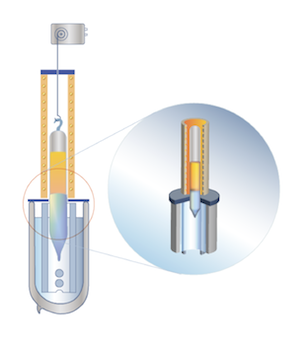
 | |
| | Vertically Directed Crystallization |
ZnSe, ZnS, ZnTe, CdSe, CdS, Bi2Te3, GaSe, PbTe, PbSe, PbS
The method is known since 1924 (I. Obreimov, L. Shoubnikov)
Crystallization is carried out in a vertical canular cylinder-shaped container. The container is cooled by compressed air. The bottom of the container is shaped as a cone to provide the conditions for “competitive growth”: only one singe crystal out of the multitude of crystals conceived at the beginning of the process “survives” and serves as the seed for the further crystal growing. The orientation of the seed predetermines the orientation of the complete crystal. The intensity of cooling of the lower part of the container regulates the speed of moving up of the crystallization front. The cylindrical shape of the container ensures the consistency of the crystal diameter.
In 1925 P. Bridgman improved the process: he introduced a different cooling method and made the container move. As the crystal grows the container goes down and gets out of the furnace. Outside of the furnace the container cools down without the forced air flow. The main advantage of Bridgman method is the possibility to control the crystallization speed.
In 1937 D. Stockbarger developed this method: he separated the spiral resistance heater into two sections, each with its own power supply. These two sections maintain the required temperature in the furnace. Between the sections there is a special ring-shaped diaphragm which keeps the temperature difference in the crystallization zone. At the beginning of the growing process the container is placed in the hot upper chamber. As the charge material melts the container goes down, passes the diaphragm and gets into the warm lower chamber.
In some modern modifications of the technique the container rotates, which helps to keep the melt blended and improves the hydro-dynamic conditions of the process.
The main advantages of this method are its simplicity and high production capacity. The main disadvantage is the contact between the crystallized material and the crucible. Since the material to be crystallized and the crucible have different thermal expansion coefficients, the crystals are usually rather stressed. Besides, the technology does not give you the possibility to control the shape and location of the crystallization front in situ, neither does it make it possible to predetermine the orientation of the crystal. And finally, the size of the crystal grown by vertical crystallization is limited.
Further modifications of this method are high-pressure vertical Bridgman method (HPVB) and high-pressure vertical zone melting (HPVZM). In HPVB method crystals grow in very high (10-100 bars) inert gas pressure. The method is widely used for growing II-VI compounds, but it has some disadvantages. The main problem is the impossibility to maintain stoichiometric composition of the crystals. The reason is that II-VI compounds evaporate and dissociate into components, and some components permeate into the crucible letting the other components shift to excess. HPVZM method solves this problem as it allows the possibility to control the composition during crystallization process.
High-pressure vertical Bridgman and high-pressure vertical zone melting methods are used for growing wide-gap II-VI semiconductors (ZnSe, ZnS, ZnTe, CdSe, CdS), chalcogenides ( Bi2Te3, Bi2Te3, GaSe, PbTe, PbSe, PbS) etc. |
 | |
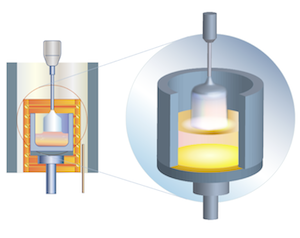
| Sapphire
The method of growing large single crystals for optical purposes was developed by S. Kyropoulos in 1926.
The seed crystal is mounted onto a seed crystal holder cooled by water and the raw material melts in the crucible. Crystallization starts when the seed contacts the melt. The crystal grows into the melt forming a hemisphere. When the crystal gets bigger and approaches the walls of the crucible the seed crystal holder together with the grown crystal rises by a few mm and the growth continues till the crystal reaches the walls of the crucible again. Due to this peculiarity as grown Kyropoulos boules have circular traces around its side surface. There are some modifications of this method, when the seed crystal holder rises continuously at a certain permanent speed. At present Kyropoulos process is almost fully automated.
The main advantage of Kyropoulos methos is that the size of the grown crystal is limited only by the size of the crucible, so the boule may be as big as 300 mm in diameter and more. Besides, due to the low temperature gradient and natural cooling the crystal possesses very low dislocation density and is almost stress-free.
Kyropoulos method is widely used for growing sapphire crystals of a very high optical quality. Kyropulos sapphire is suitable for manufacturing ingots and substrates for LED and RF applications, windows, lenses, any kind of precision optics, watch glasses etc.
|
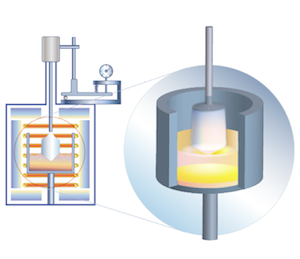 | |
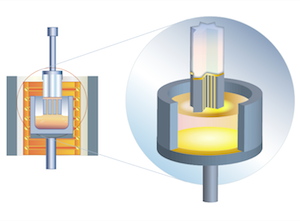
| | Stepanov (Edge-Defined Film-Fed Growth, EFG) Method |
Sapphire, ruby
The method is known since 1965.
The principle of EFG technology is that crystals are grown from the melt film formed on top of a capillary die. The melt rises from the crystallization front within the capillary channel. The growth speed is 1 to 4 cm/hour in inert medium (argon). The method makes it possible to grow crystals of complicated shape.
With the help of an automated computer system the weight, shape and quality of the crystals are constantly controlled during the growth process.
Stepanov (or EFG) method is used for growing sapphire of any given shape, including tubes, rods, sheets, and fibers. EFG technology makes it possible to get unique shapes and sealed assemblies. It is possible to grow ribbons up to 300 mm in length and 60 mm in width, or tubes and crucibles with OD up to 50 mm. Crystals grown by this method can have different crystallographic orientations (A, C, random) and are mainly used for industrial, scientific, medical, mechanical and standard optical applications (tubes for CVD reactors, arc envelopes for vapour lamps, nozzles, insulators, high-pressure reactors, high-vacuum equipment, cryogenic equipment).
|
 | |
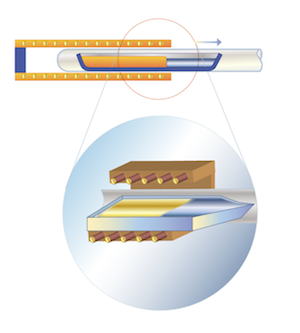
| | Bagdasarov (Horizontally Directed Crystallization) Method |
Sapphire, ruby
The method was developed in 1975.
Crystals are grown from the melt in a horizontal boat-shaped container at the speed of 8-10 mm per hour. This method makes it possible to get large slabs with almost perfect edges and of any given crystallographic orientation: C-plane, M-plane, R-plane or A-plane. Typical crystals grown by this method are shaped as thick rectangles (e.g. 220mm x 250 mm x 25-35 mm).
One of the advantages of this method is its relative technological simplicity. Besides HDC technique makes it possible to recrystallize the raw material before growth and remove most of impurities.
HDC method is used for growing sapphire and ruby single crystals. The fact that the surface of the melt is very wide compared to its thickness makes it easy to add any required dopant during the crystallization process.
Bagdasarov sapphire has a very high optical quality and is suitable for very demanding optical, mechanical, RF and LED applications. This material is ideal for manufacturing Ingots, blanks and windows of diameters 2”-8” and thickness >3 mm. |
 | |
| | |
|
Growth from Solutions
The peculiarity of this group of technologies is that the chemical composition of the finished crystal is considerably different from the composition of the feed liquid. Water, multicomponent water solutions, melts of some chemical substances may serve as solvents. Depending on the process temperature and the chemical composition of the solvent this group may be divided into low-temperature water solutions (80-90 deg. C), high-temperature water solutions (hydrothermal method, temperatures are over 800 deg. C), salt melts (crystallization from solution in melt, temperatures up to 1500 deg. C).
Crystallization from solutions is used to grow materials which resolve at temperatures below their melting point and to grow crystals that have several polymorphous modifications. The growing process is carried out at temperatures below the melting point and as a result, crystals grown from solutions have no defects typical for crystals grown from the melt. The driving force of the crystallization process is supersaturation. The level of supersaturation is characterized by the value of superfusion (supercooling). To crystallize the material you can change the temperature of the solution, or change the composition of the solution, or use a chemical reaction.
Crystallization from Low-Temperature Solutions
KDP
This technology has a few modifications described below.
The principle of low-temperature water solution method is that the supersaturation is achieved by lowering the temperature of the solution in the crystallization zone. This method is adopted for growing potassium-sodium tartrate (seignette salt), tryglicine sulfate, potassium aluminium sulfate.
The method of temperature gradient consists in creating two zones with different temperatures. One zone is used for dissolving the substance, the other is used for crystallization. The simplest example of this structure is a tall reservoir with a feed liquid in its lower part and a seed crystal in its upper part. Due to the thermosolutal convection the matter is continuously transferred from the lower part of the reservoir to its upper part, i.e. to the crystallization zone. This technology is used for growing potassium dihydrogen phosphate (KDP) crystals. The speed of growth is about 1 mm per day and it takes up to 2 months to get a crystal weighing 400 g.
In the process of concentrated convection the exchange between the solution zone and the crystallization zone is driven by the difference in density values of the saturated and non-saturated solutions. The seed crystal in mounted in the lower part of the reservoir, and the feed liquid is placed in the upper part of the reservoir. Because the temperature in the upper part of the vessel is higher than in its lower part, the convection is suppressed, the saturated solution with a higher density gets down, becomes supersaturated and the crystallization process starts.
The vaporization technology requires constant temperature and isothermal conditions. The solvent evaporates, which results in supersaturation of the solution. The level of saturation is regulated by the growing crystal. The biggest drawback of this method is a very low speed of crystallization.
The use of chemical reactions for growing crystals is based on the following process: the dissolved components interreact and as a result, a solid matter disengages. The method can be used if the solubility of the grown crystal is lower than the solubility of the feed components. As a rule, the rate of chemical reactions in solution is very high and a lot of small crystals evolve very fast.
Electrochemical crystallization consists in electrolytic process and is used for growing metal crystals.
Hydrothermal Crystallization
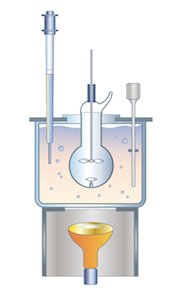 The characteristic features of this method are water solution, high temperatures (over 100 deg. C), high pressure (over atmospheric pressure), the use of mineralizing agent (a substance with high solubility). The combination of these conditions makes it possible to dissolve the substance which is to be crystallized and achieve the required supersaturation. The method is very effective for growing crystals at temperatures far below their melting point. For example, it is impossible to grow sphalerite (cubic zinc-blende) ZnS from the melt, because at the temperature of 1080 deg. C the crystal undergoes polymorphous transformation and becomes a wurtzite (a hexagonal crystal). In hydrothermal conditions ZnS can be crystallized at temperatures of 300-500 deg. C, i.e. the cubic (sphalerite) modification is sustained. The characteristic features of this method are water solution, high temperatures (over 100 deg. C), high pressure (over atmospheric pressure), the use of mineralizing agent (a substance with high solubility). The combination of these conditions makes it possible to dissolve the substance which is to be crystallized and achieve the required supersaturation. The method is very effective for growing crystals at temperatures far below their melting point. For example, it is impossible to grow sphalerite (cubic zinc-blende) ZnS from the melt, because at the temperature of 1080 deg. C the crystal undergoes polymorphous transformation and becomes a wurtzite (a hexagonal crystal). In hydrothermal conditions ZnS can be crystallized at temperatures of 300-500 deg. C, i.e. the cubic (sphalerite) modification is sustained.
Hydrothermal method is used for growing quartz, ruby, calcite crystals etc. Because the process is carried out at relatively low temperatures the finished crystals have almost no stress, no plastic deformations, no blocks and other structural defects.
Crystallization from Solution in Melt
The technology makes use of the high solubility of some heat-resistant materials in liquid mineral salts and oxides. The process is carried out in air at the temperature equal to the melting point of the crystallized material. The method is used for growing yttrium iron garnet, barium titanate etc.
Zone crystallization from solution in melt (or zone recrystallization by temperature gradient, or zone melting with a solvent) is a process similar to float-zone technology. Due to the temperature gradient the narrow zone of solution travels along the sample. Between the seed crystal and the sample there is a thin layer of a solid substance which serves as the solvent.
Crystallization from Vapor
CVD Zinc Selenide
This group of methods is used for growing crystals, epitaxial layers, thin films etc. The methods are based either on the condensation of the crystallized substance or on a chemical reaction.
Condensation of Vapor
The technologies are subdivided according to the method of delivery of the substance in the form of vapor to the growing crystal.
In molecular beam epitaxy a compact source heated to a very high temperature emits atoms or molecules which get onto the substrate where the material condensates.
The method of cathode sputtering uses a glow-discharge between the cathode (which emits the electrons of the sputtering material) and the anode (where the substrates are placed).
|
| CVD Zinc Selenide - Growth Methods |
Chemical Reaction
The technologies are classified according to the type of chemical reaction used in crystallization process.
In methods of chemical delivery the process starts when the substance to be crystallized (in either liquid or solid state) interreacts with other substances and forms a gas. The gas is transferred to the zone with a different temperature, where it resolves by a counter reaction and extricates the initial substance.
In methods of chemical decompounding a gas is put into the crystallization zone. Under certain conditions the gas decomposes and extricates the substance which is to be crystallized.
In the method of chemical synthesis the substance to be crystallized evolves as a result of the chemical reaction between the gas components in the crystallization zone.
An example of chemical synthesis is the growth of polycrystalline ZnSe by chemical vapor deposition. ZnSe vapor received from the chemical reaction between Zn and Se is deposited on the walls of the container forming four plates merged perpendicularly. The maximal thickness of such a plate is 15 -16 mm, the maximal width is 102-106 mm and the efficient (usable) length amounts to 200-250 mm
|
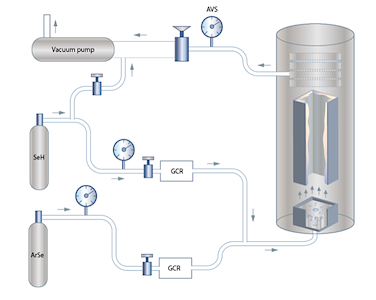 | As a result of the synthesis we get four ZnSe plates merged perpendicularly. Maximum side thickness amounts to 15-16 mm, width to 102-106 mm, and efficient length to 200-250 mm. |
| |
|
|
|
 The characteristic features of this method are water solution, high temperatures (over 100 deg. C), high pressure (over atmospheric pressure), the use of mineralizing agent (a substance with high solubility). The combination of these conditions makes it possible to dissolve the substance which is to be crystallized and achieve the required supersaturation. The method is very effective for growing crystals at temperatures far below their melting point. For example, it is impossible to grow sphalerite (cubic zinc-blende) ZnS from the melt, because at the temperature of 1080 deg. C the crystal undergoes polymorphous transformation and becomes a wurtzite (a hexagonal crystal). In hydrothermal conditions ZnS can be crystallized at temperatures of 300-500 deg. C, i.e. the cubic (sphalerite) modification is sustained.
The characteristic features of this method are water solution, high temperatures (over 100 deg. C), high pressure (over atmospheric pressure), the use of mineralizing agent (a substance with high solubility). The combination of these conditions makes it possible to dissolve the substance which is to be crystallized and achieve the required supersaturation. The method is very effective for growing crystals at temperatures far below their melting point. For example, it is impossible to grow sphalerite (cubic zinc-blende) ZnS from the melt, because at the temperature of 1080 deg. C the crystal undergoes polymorphous transformation and becomes a wurtzite (a hexagonal crystal). In hydrothermal conditions ZnS can be crystallized at temperatures of 300-500 deg. C, i.e. the cubic (sphalerite) modification is sustained.